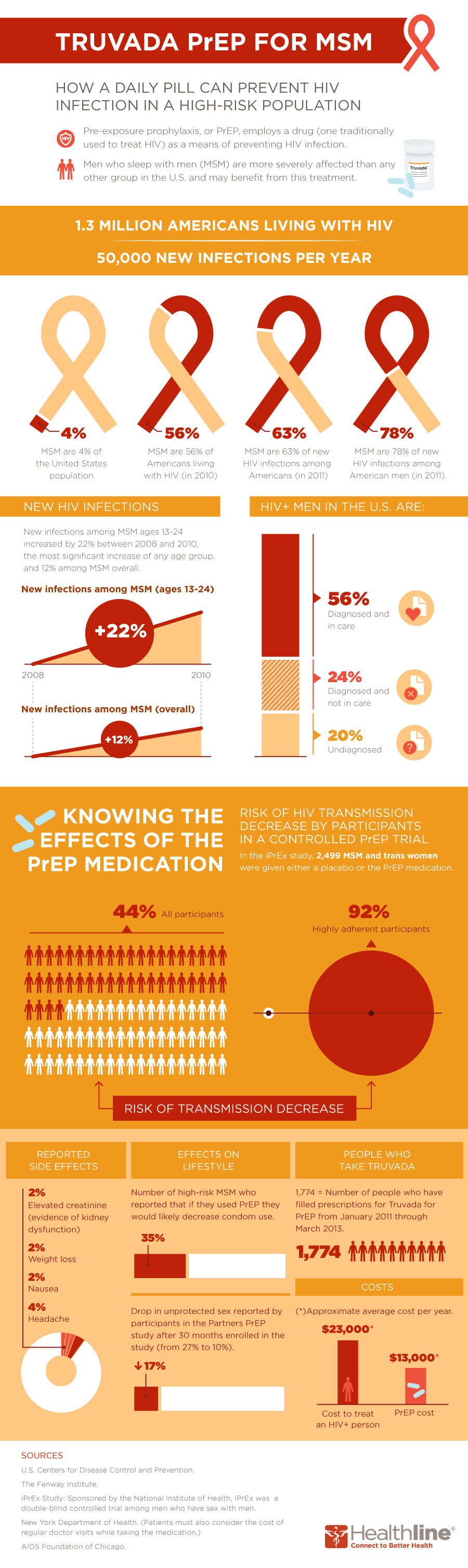
Truvada is the first drug approved by the FDA for the prevention of HIV infection. Known as PrEP (short for pre-exposure prophylaxis), this treatment is meant to be employed alongside other risk-reduction measures, such as safer sex and regular HIV testing; however, it has caused some controversy, both among medical experts and in communities of men who sleep with men—the group hardest hit by HIV in the United States.
Here’s a look at some of the numbers (for the full story, read "Truvada for PrEP: Experts Weigh In on the Newest Way to Prevent HIV/AIDS").
healthline.comOther proponents argue:
“Condoms are more effective than PrEP”
Condoms should remain the gold standard – they protect against STDs and HIV when use consistently. However, we have a hard time admitting that there are young men who are not using condoms at all for varying reasons. That is why this prevention pill is marketed as a medication for “high risk” individuals – commercial sex workers, who may negotiate no condom usage for more pay or may not be able to negotiate condom use at all, HIV negative men and women in relationships with someone who is HIV positive, and people who inject drugs and share needles, often because they don’t have access to clean syringes. Or, maybe gay men who don’t like the feel of a condom so they inconsistently use them. Truvada as PrEP caters to them.
In a recent study, among users who took the medication consistently, as indicated by drug levels, Truvada was found to be 92 percent effective against the transmission of HIV. For the most protection, PrEP users need to adhere to a daily regimen.
The bottom line: both PrEP and condoms are highly effective if you use them consistently and correctly. See more HERE










.mkv_000108708.jpg)
.mkv_000109059.jpg)
.mkv_000113988.jpg)












































+(Light).jpg)











































0 comments:
Post a Comment